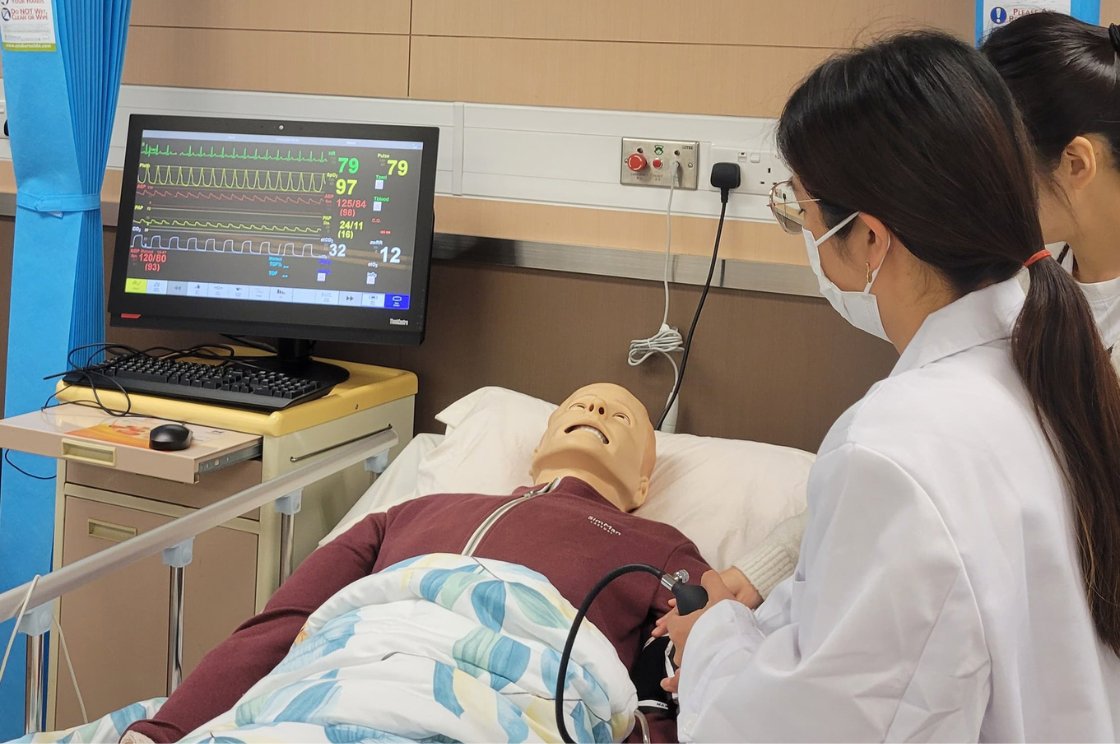
VTC students/graduates (Diploma of Foundation Studies (DFS) / Diploma of Vocational Education (DVE) / Diploma of Vocational Baccalaureate (DVB)) applying for Full-time Higher Diploma programmes should pay attention to the following admission procedures:
- Login to the "VTC S6 Admission Portal" / "VTC Articulation Portal" to check your finalised offer results on 4 Jul 2025 (12:00 noon);
- If you are given a Firm Offer, you should download the payment advice and offer documents, pay the registration fee and upload the registration fee payment receipt to complete the registration by 9 Jul (12:00 noon).